Chemical bonds are responsible for the formation of all existing compounds on the planet, they are what guarantee the stability and greater diversification of compounds, guarantee the emergence of new properties, new applications.
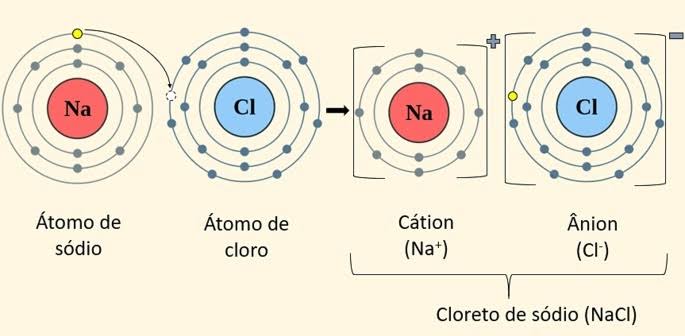
Ionic: Chlorine has seven electrons in the outermost shell, and sodium has one, when these two elements meet, the seven electrons of chlorine “take out” the last electron from sodium. Two ions then appear: chlorine (Cl-) and sodium (Na+), which remain united by the attraction of opposite charges. This union forms an ionic compound. They are non-metals and metals.
Cl= Chlorine; Na= Sodium
Covalents: happens between atoms that tend to gain electrons. But it is not possible for everyone to win, they are made by sharing electron pairs, that is, they “borrow” one, two, three, or four electrons. They are non-metals.
H= Hydrogen; Cl= Chlorine
Metallic: happens between metal atoms that have a tendency to lose electrons and, when they unite, form a crystalline lattice, it is caused by the appearance of a “cloud of free electrons”, involves the atoms and does not belong to any of them in particular. Are metals
Na= Sodium
Autores: Júlia Marques Seixas; Ana Avais de Mello; Mateus Aquino Postiglioni; Henrique Scorsim dos Santos; William Chesini
Fonte da imagem: SAE Apostila
https://www.todamateria.com.br/ligaces-quimicas/ Acesso: 24 de junho de 2021.